Frontier Science Partners With SUNY Stony Brook On cCMV Study
Frontier Science is proud to announce its partnership with SUNY Stony Brook and New York State on the Prospective Observational Study of Asymptomatic cCMV Transmission to Infants for Virological Evaluation in New York State (PROACTIVE NYS). Frontier Science serves as the Statistical and Data Management Center, providing a wide range of technical services and support for the data management and statistical needs of the project.
Frontier Science Collaborates with Rutgers, Vanderbilt, and Johns Hopkins on Tuberculosis Report International Coordinating Center (TB-RICC)
Frontier Science is proud to collaborate on a $20 million award from the National Institute ofAllergy and Infectious Diseases (NIAID). The purpose of this award is to support and coordinate research conducted on tuberculosis (TB) control and prevention through the TB-RePORT (Regional Prospective Observational Research in Tuberculosis) International (TBRI) and its networks.
Redesigned Precautionary and Prohibited Medications Database
The ACTG and IMPAACT Data Management Center at Frontier Science is pleased to announce the release of the redesigned Precautionary and Prohibited Medications Database (PPMD).
CASCADE Network Featured in Recent White House Press Release
In December 2022, a White House press release highlighted the launch of the HIV/Cervical Cancer Prevention ‘CASCADE’ Clinical Trials Network by the National Cancer Institute (NCI). Frontier Science is the prime awardee for the CASCADE Network Coordinating Center (CNCC).
Frontier Science Receives Global HIV/Cervical Cancer Coordinating Center Prime Award From The NCI
Frontier Science & Technology Research Foundation is the proud recipient of a five-year, $7.1 million dollar award from the National Cancer Institute (NCI) Division of Cancer Prevention (DCP) of the National Institutes of Health (NIH). The award will be used to set up and manage the coordinating center for the HIV/Cervical Cancer Prevention ‘CASCADE’ Clinical Trials Network.
CPQA Project Renewal
The National Institutes of Health (NIH) has awarded $12.8 million to the University at Buffalo School of Pharmacy and Pharmaceutical Sciences to lead a clinical pharmacology quality assurance program for NIH-funded laboratories and research networks across the globe conducting HIV and infectious disease research. This award also renews a longstanding subcontract with Frontier Science, which has supported the program’s data management and analytics for the past seven years.
Medidata Rave and Frontier Science Case Study: Remote Source Review
In May 2020, when Frontier Science suddenly needed to implement remote study monitoring, they turned to Medidata for a technology solution. Together they implemented Remote Source Review (RSR) in just four months.
IMPAACT P1107 Team Presents First Known Case of a Woman with HIV Remission
At CROI 2022, the IMPAACT P1107 protocol team reported the first known case of a woman with HIV remission following a CCR5Δ32 stem cell transplant for treatment of acute myelogenous leukemia.
C3PNO Virtual Data Repository Stimulates Data Sharing in a Consortium
The Collaborating Consortium of Cohorts Producing NIDA Opportunities (C3PNO), a joint project between UCLA and Frontier Science, recently published their VIrtual Data Repository work in the Online Journal of Public Health Informatics. This article specifically showcased the work of the Frontier Science team and highlights the importance of data sharing.
OlympiA Phase III Results Published in The New England Journal of Medicine show significantly longer survival with olaparib (Lynparza)
OlympiA Phase III Results titled “Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer” were published in the June 3, 2021 edition of The New England Journal of Medicine.
Results of this analysis will also be presented during the plenary session of the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting (abstract LBA#1). The results were made available by ASCO on 3rd June and simultaneously published in The New England Journal of Medicine prior to their presentation in the plenary session. An estimated 2.3 million people were diagnosed with breast cancer worldwide in 2020, and BRCA1 and BRCA2 mutations are found in approximately 5% of breast cancer patients.
Labelscape: A Mobile App for Creating and Printing Specimen Labels
Frontier Science is pleased to announce the release of Labelscape, a new Android and iOS app for creating and printing specimen labels.
OlympiA Phase III trial of Lynparza in the adjuvant treatment of BRCA-mutated HER2-negative high-risk early breast cancer to be analysed and reported early
The OlympiA Phase III trial of AstraZeneca and MSD’s Lynparza (Olaparib) will move to early primary analysis and reporting following a recommendation from the Independent Data Monitoring Committee (IDMC).
Based on the planned interim analysis, the IDMC concluded that the trial crossed the superiority boundary for its primary endpoint of invasive disease-free survival (iDFS) and demonstrated a sustainable, clinically relevant treatment effect for Lynparza versus placebo for patients with germline BRCA-mutated (gBRCAm) high-risk human epidermal growth factor receptor 2 (HER2)-negative early breast cancer, and recommend primary analysis now take place.
Frontier Science Foundation Welcomes Marlene Smurzynski, PhD, MSPH
Frontier Science Foundation welcomes Marlene Smurzynski, PhD, MSPH, as our new Executive Director. Marlene joins our Frontier Science Leadership Group with an initial focus on assisting with business development efforts and other infrastructure needs. Marlene brings with her vast experience and knowledge gained from working in the private and public sector. Following receipt of her doctorate in Epidemiology from UNC Gilling’s School of Global Public Health, she joined the Harvard T.H. Chan School of Public Health as a research scientist working with the AIDS Clinical Trials Group. Following her work at Harvard, Marlene launched and thereafter directed a new division in observational research at a large CRO.
NIH Announces Restructured HIV Clinical Trials Networks
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has announced the clinical investigators and institutions that will lead four NIH HIV clinical trials networks over the next seven years to conduct the innovative, efficient clinical research needed to accelerate progress against the HIV pandemic.
IMPAACT 2007 Pharmacokinetic and Safety Data Informs U.S. FDA
The IMPAACT Operations Center has announced that IMPAACT 2007 data informed the FDA approval of two supplements for Selzentry (Maraviroc) Tablets and Oral Solution, which expanded the Selzentry label to include dosing for individuals less than two years of age and weighing at least 2 kg.
IMPAACT P1093 Informs U.S. FDA Decision
The IMPAACT Operations Center has announced that data from IMPAACT P1093 and the ODYSSEY (PENTA20) study have informed the United States (U.S.) Food and Drug Administration (FDA) decision to approve the first-ever dispersible tablet formulation of Tivicay PD (dolutegravir) tablets for children as young as four weeks of age and weighing at least 3 kg (or about 6.5 pounds). Prior to this approval, dolutegravir was indicated in the U.S. for children from six years of age and weighing more than 30 kg (or about 66 pounds).
Psychometric Data Linking Using C3PNO Data
A study using data from the Collaborating Consortium of Cohorts Producing NIDA Opportunities (C3PNO) cohorts demonstrates how data capturing similar depression constructs in HIV-risk populations can be linked to a common depression metric. Taking advantage of previous linking studies with multiple measures of depression measures, the study illustrates how to take these results, Item Response Theory (IRT) item parameters, and apply them in the service of data harmonization across C3PNO cohorts in HIV/substance use populations.
Buffalo Companies Attacking COVID-19
Frontier Science is one of several Buffalo companies and research institutions who are collaborating with worldwide partners to test and treat coronavirus, featured in the April 10, 2020 edition of the Buffalo Business First journal.
Frontier Science Response to COVID-19: A Message From The CEO
Frontier Science is first and foremost an organization committed to advancing medical research. As such, we understand the potential effects of public health crises such as the one we are currently facing with COVID-19, and we also understand the resulting necessity for all organizations and businesses to appropriately respond to this public emergency in a swift and deliberate manner. Frontier Science, along with all other organizations, must respond quickly to ensure the safety of our employees, collaborators, sponsors, and clients. As the situation around COVID-19 continues to evolve I want to take a moment to share a few critical updates.
Employee Spotlight: Stephen Hart, PhD, Senior Research Scientist
When Dr. Hart joined Frontier Science Foundation in 1998, he brought with him over two decades of both qualitative and quantitative research experience in several areas of social scientific research. As a Senior Research Scientist at Frontier Science, Steve has conceptualized, developed, and directed the use of systems - human and automated - for receiving, checking, storing, and analyzing viral genetic sequences, especially of HIV-1.
NIAID News Release Features IMPAACT P1104s Study Results
IMPAACT Study P1104s, published December 18, 2019 in the online journal Clinical Infectious Diseases, found that children who acquired HIV in utero or during birth or breastfeeding did not perform as well on tests measuring cognitive ability, motor function and attention as their peers who do not have HIV. The publication prompted a News Release in the NIAID News & Events Newsroom titled “Children with HIV Score Below HIV-Negative Peers in Cognitive, Motor Function Tests.” This disparity worsens over time despite early HIV treatment, the NIH study finds.
The National Cancer Institute awards a $12 million grant to the University of Wisconsin and Frontier Science Foundation
Frontier Science Foundation, in partnership with the University of Wisconsin-Madison (UW), has been awarded a large five-year project from the National Cancer Institute (NCI) as a Data Management, Auditing, and Coordinating Center (DMACC) for the Cancer Prevention Clinical Trials Network (CP-CTNet) in the United States. KyungMann Kim, PhD, professor, Department of Biostatistics and Medical Informatics at the University of Wisconsin School of Medicine and Public Health, will lead the DMACC in the UW Department of Biostatistics and Medical Informatics. Frontier Science Foundation, headquartered in Amherst, New York, brings its decades of cancer research experience and its exceptional data management expertise into this large US-based initiative to provide data management and research support.
IMPAACT P1078 Study Results Published in The New England Journal of Medicine
A clinical trial funded by the National Institutes of Health has studied the safety of treatment with the antibiotic isoniazid to prevent tuberculosis (TB) in women who are living with HIV, are pregnant or have recently given birth, are taking antiretroviral therapy (ART), and live where TB is highly prevalent. The trial found that whether the treatment was begun during pregnancy or 12 weeks after delivery, it was similarly safe for the women. However, there was significantly greater risk of poor health outcomes and death for the fetuses and newborns of these women if isoniazid preventive therapy began during pregnancy than if it began 12 weeks after delivery. This finding is concerning and merits research into alternative approaches to TB preventive therapy in pregnant women, according to the study investigators. These findings are reported in the Oct. 3 issue of The New England Journal of Medicine.
Employee Spotlight: Ting-Li Lin, PhD, Research Scientist
Ting-Li Lin joined Frontier Science Foundation Madison office as a Research Scientist in 2017. He serves as a scientific lead for the Madison office on statistical related matters, and is involved in reviewing, producing, and presenting IDMC monitoring reports. He assists the Madison office director in advising the staff statisticians and overseeing the statistical operations of the Madison office, and provides statistical support to other Frontier Science offices as needed.
IMPAACT Gender Identity Assessment: Site Survey Results & Implementation
At the IMPAACT Network Annual Meeting held in Washington, DC June 2019, Laura Smith, MPH, IMPAACT Chief Data Manager, co-presented a poster entitled IMPAACT Gender Identity Assessment: Site Survey Results & Implementation along with colleagues Susannah Allison, PhD, Miriam Chernoff PhD, and Rona Siskind, MHS.
Sue Siminski Named CEO At Frontier Science Foundation
The Frontier Science Foundation Board of Directors has approved a new Chief Executive Officer position and has named Sue Siminski, currently the Foundation’s Executive Director, as the Foundation’s Chief Executive Officer (CEO) effective July 1st.
C3PNO Virtual Data Repository Search Tool Is Available For Use
The Collaborating Consortium of Cohorts Producing NIDA Opportunities (C3PNO) is pleased to announce the launch of its Virtual Data Repository. C3PNO is the coordinating center for NIDA longitudinal cohorts that represent a combined sample size of about 12,000 active and 20,000 historical participants. C3PNO links behavioral and biological data from cohorts spanning the United States and Canada that follow a diverse group of high risk HIV-negative and HIV-positive persons in and out of care.
Dr. Kathryn P. Gray Joins Frontier Science Foundation
Dr. Kathryn P. Gray joined Frontier Science Foundation as Director of Biostatistics and Boston Office Director on March 1, 2019. Dr. Gray has been working in collaborative cancer clinical research, with the focus on gynecologic, breast, renal and prostate cancers, since 2010. She enjoys collaborating with investigators from multiple disciplines on clinical trial and translational studies with the goal of developing personalized cancer treatments.
PHIA 2 Project To Build On The PHIA Project Achievements
Frontier Science began its PHIA collaboration with ICAP at Columbia University in 2015 by providing LDMS installations, user support, training, and central database services across 14 mostly sub-Saharan countries in support of the Population-based HIV Assessment (PHIA) project. These countries include: Cameroon, Cote d’Ivoire, Ethiopia, Haiti, Kenya, Lesotho, Malawi, Namibia, Rwanda, Swaziland, Tanzania, Uganda, Zambia, and Zimbabwe. PHIA surveys are implemented under the leadership of each country’s Ministry of Health and by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), CDC, and by ICAP at Columbia University. In 2017 Frontier Science also began a collaboration with the University of Maryland School of Medicine on the PHIA Nigeria study (NAIIS).
Time To Abandon Early Detection Cancer Screening
Michael Bretthauer, MD, PhD, President, Frontier Science, Frontier board members Hans-Olov Adami and Mette Kalager, along with their colleagues Unnur Valdimarsdottir and John P.A. Loannidis have published an editorial in the European Society for Clinical Investigation titled “Time to abandon early detection cancer screening.”
Employee Spotlight: Howard Gutzman, LDMS Program Director
Howard Gutzman, Program Director for the Laboratory Data Management System (LDMS), has worked at Frontier Science for fourteen years. Howard began his career at Frontier Science as a lab data manager for the Adult and International Maternal Pediatric Adolescent HIV Clinical Trials networks and was a member of the LDMS training team. Within three years, Howard was promoted to a Project Manager within Frontier’s lab division and took on additional responsibilities in overseeing the LDMS related projects.
How To Get Your Research Published In The Best Journals
A 2-day course on scientific writing in medicine, “How To Get Your Research Published In The Best Journals” has been scheduled for 13-14 May 2019 in Oslo, Norway. The course is intended for clinicians and researchers with some experience in medical writing or with an interest in learning to write or review scientific papers. Researchers who want to get results published in a respected scientific journal will learn how to prepare a manuscript in order to increase its chance of being accepted for publication in leading journals.
Utility of the National Death Index in the Pediatric HIV/AIDS Cohort Study
The Journal of Acquired Immune Deficiency Syndrome (JAIDS) recently published an article from investigators of the Pediatric HIV/AIDS Cohort Study (PHACS) that outlines the researchers’ experience using the National Death Index (NDI) to identify if any lost to follow-up study participants occurred due to death among the 4,207 participants enrolled in two PHACS cohorts.
Frontier Science Welcomes Two Harvard Fullbright Students
Frontier Science is pleased to welcome two Harvard Fulbright students this year, Lise Helsingen, MD and Paulina Wieszczy as affiliate researchers in our Brookline office.
Jenna Kearly, MPH, Presents At Medidata NEXT
Jenna Kearly, EDC Study Build Project Lead for Frontier Science, presented at the Medidata NEXT meeting held in NYC Oct 24-25, 2018.
When No Guideline Recommendation Is The Best Recommendation In Clinical Practice
The core of clinical practice guidelines is the recommendations, and the ideal situation for guidelines is an unequivocal body of evidence about the benefits and harms of different treatment options, and related costs and resources. However, when high quality evidence is lacking, a no guideline recommendation is the best recommendation in clinical practice, according to co-authors Michael Bretthauer, MD, PhD and Mette Kalager, MD, PhD, who published their comments in the September 15th edition of THE LANCET.
Dr. Soyeon Kim Published in the New England Journal of Medicine (NEJM)
Dr. Soyeon Kim, a Senior Research Scientist at Frontier Science Boston, is co-author on an original article, Bacterial Factors in Relapse after Tuberculosis Therapy, published in the August 30, 2018 edition of the New England Journal of Medicine.
Dr. Hart Published in the Journal of Acquired Immune Deficiency Syndromes (JAIDS)
Stephen Hart, PhD, a Senior Research Scientist at Frontier Science Foundation (FSF), is the lead author of an article published in the September 1, 2018 edition of the Journal of Acquired Immune Deficiency Syndromes (JAIDS), “Impact of changes over time in the Stanford University genotypic resistance algorithm.” The article comes out of ACTG Data Analysis Concept Sheet (DACS) 330, which Steve designed and led. Sarah Strobino, BS, also from Frontier Science Foundation, made major contributions to the project. Saran Vardhanabhuti, PhD and Linda J. Harrison, MSc, from Harvard T.H. Chan School of Public Health, did the trend analysis.
IAS Conference Review
The 10th International Workshop on HIV Pediatrics, held July 20 to 21, 2018 in Amsterdam, The Netherlands, is entirely devoted to research in prevention and treatment of HIV infections in infants, children and adolescents, making it a premier forum for the world’s leading experts in pediatric, adolescent and maternal HIV research. The 22nd International AIDS Conference, also held in Amsterdam, from July 23 to 27, is the world’s largest conference on AIDS, biennially bringing together leading experts to advance knowledge about HIV. Several networks with whom Frontier Science collaborates, including the IMPAACT, ACTG, and PHACS networks, were highlighted in Amsterdam this year, and Frontier Science was acknowledged as one of the affiliated organizations working on the HIV studies.
Employee Spotlight: Dr. Soyeon Kim
Dr. Soyeon Kim, Senior Research Scientist, joined Frontier Science Foundation Boston office in September 2017. Soyeon has worked in the design, analysis, monitoring, and reporting of clinical research studies with International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) and its predecessor Pediatric AIDS Clinical Trials Network, as well as investigator-initiated projects, for more than 20 years. She enjoys working in multidisciplinary teams, closely collaborating with investigators to design trials of clinical and public health significance.
Frontier Science Leadership and Development Announcement
Michael Bretthauer, Frontier Science Foundation President, has announced key features of a new business development plan for Frontier Science in the coming years that will maintain and develop current business platforms and areas, and explore and establish more diverse funding sources and collaborations. The first step in this future development plan is the establishment of a leadership structure to meet the opportunities ahead, including a new Executive Director.
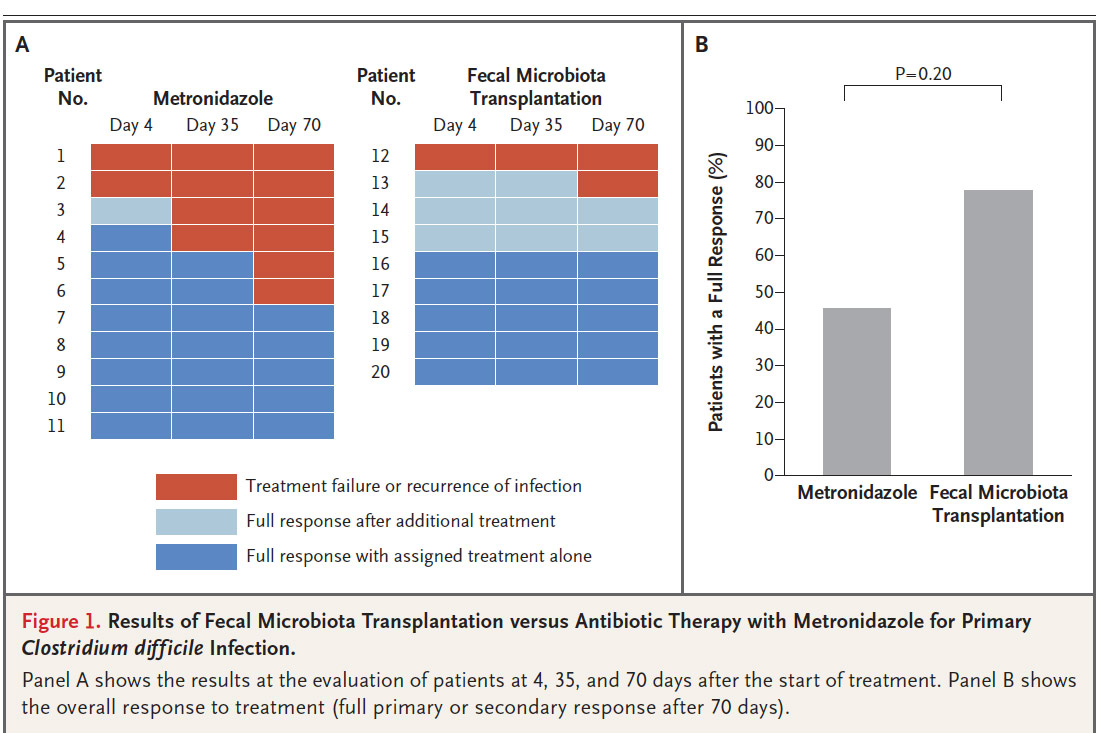
Norwegian Pilot Study Published In The New England Journal of Medicine
A Norwegian pilot study published in the June 2, 2018 edition of the New England Journal of Medicine shows that a supply of intestinal bacteria can be better than antibiotics for all patients with Clostridium difficile Infection, including those who have it for the first time. The study, Fecal Microbiota Transplantation for Primary Clostridial Difficile Infection examined whether the intestinal bacterial infections of a healthy person (fecal microbial transplantation) can restore a healthy and resilient intestinal flora so that the patient with C Diff infection can get rid of the infection without resorting to antibiotics.
Frontier Science Poster Presentations at the Society For Clinical Trials Annual Meeting
Frontier Science Amherst staff presented two posters at the Society For Clinical Trials 2018 annual meeting. A Unique Collaboration for a Data Coordinating Center outlines the INVESTED collaboration between the University of Wisconsin-Madison and the Frontier Science Data Management Center. Calculating Antiretroviral Drug Resistance: An Innovative Open-Source Tool showcases an antiretroviral drug resistance calculations tool developed at Frontier Science that uses customizable algorithms.
IAS Pre-Conference: Research Opportunities Using the C3PNO Virtual Repository
The Collaborating Consortium of Cohorts Producing NIDA Opportunities (C3PNO) invite you to attend an IAS pre-conference in Amsterdam, the Netherlands, on July 22 from 12-2pm CET entitled Blood, Guts & Glory: HIV & Substance Use Research Opportunities Using the C3PNO Virtual Repository to Link NIDA Cohort Data.
C3PNO, the data coordinating center for the National Institute on Drug Abuse (NIDA) longitudinal HIV cohorts, will demonstrate and discuss opportunities related to the new C3PNO virtual repository which links laboratory, clinical, substance use, behavioral, and biological data. The C3PNO goal is to stimulate research with outside investigators and encourage new collaborations with international cohorts studying substance use in the context of HIV pathogenesis. Representatives from Frontier Science will include: Dr. Soyeon Kim, Senior Biostatistician, Adina Stoica, Software Project Manager and Sue Siminski, Co-Principal Investigator.
Original Research Results Published in the Annals of Internal Medicine
Members of the Norwegian Colorectal Cancer Prevention Study Group (NORCCAP), including Michael Bretthauer, Frontier Science President, and Mette Kalager, MD, PhD, Board Member, have authored a study published April 24, 2018 in the Annals of Internal Medicine, Long-Term Effectiveness of Sigmoidoscopy Screening on Colorectal Cancer Incidence and Mortality in Women and Men: A randomized trial. The study objective is to determine the effectiveness of flexible sigmoidoscopy screening after 15 years of follow-up in women and men.
New ISC Website Highlights Frontier Science Independent Statistical Center
The Frontier Science Independent Statistical Center, or ISC, has launched a new website at http://www.fstrf-isc.org. The website outlines the Frontier Science ISC areas of expertise and experience, and the graphical reporting style of the ISC.
Frontier Science and UCLA Partner to Stimulate Research on AIDS and Substance Abuse
Frontier Science is pleased to announce C3PNO, a partnership with the University of California, Los Angeles (UCLA) to stimulate new research in the areas of HIV/AIDS infection in the context of substance abuse.
Employee Spotlight: Laurie Dooley Butler
Laurie Dooley Butler, Data Manager for the Pediatric HIV/AIDS Cohort Study (PHACS)
Our “Employee Spotlight” shines on Laurie Dooley Butler, Data Manager for the Pediatric HIV/AIDS Cohort Study (PHACS). Laurie has worked at Frontier Science for fourteen years. She has an undergraduate degree in Medical Technology from Daemen College, and an MBA from the State University of New York at Buffalo.
2018 DFCI/FSTRF Marvin Zelen Memorial Symposium
Announcing the 2018 DFCI/FSTRF Marvin Zelen Memorial Symposium, to be held on Friday April 6, 2018 from 1:00-6:00pm at the Dana-Farber Cancer Institute.
The topic this year is Data Science in Biomedical Research.
Speakers for this year include:
- Barbara Englehardt, Ph.D., Assistant Professor, Department of Computer Science, Princeton University
- Jeffrey Leek, Ph.D., Associate Professor, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health
- Steve Goodman, M.D., MHS, Ph.D., Professor of Medicine and of Health Research and Policy, Stanford University
- JJ Allaire, Founder and CEO of RStudio
- Fernanda Viegas, Co-leader, Google’s “Big Picture” Data Visualization Group
- Jayashree Kalpathy-Cramer, Associate Professor of Radiology, Massachusetts General Hospital
Dr. Meredith Regan Visits Frontier Science To Present IBCSG Trial Results
Meredith Regan, Sc.D., Director of the Statistical and Data Management Center for the International Breast Cancer Study Group (IBCSG) and co-lead for the IBCSG TEXT and SOFT Trials, visited Frontier Science to present the results of several trials. These results were recently presented during the San Antonio Breast Cancer Symposium (SABCS 2017).
Jamie Hoel Named Madison Office Director
Jamie Hoel, MS, has been named the new Director of the Madison Office of Frontier Science, President Michael Bretthauer announced today. “I am absolutely convinced that Jamie will fill this important role with success, and ask you to welcome him and support him the best you can in his new position,” said Dr. Bretthauer.
Employee Spotlight: Lynette Blacher
Lynette Blacher, Director of Data Management for the International Breast Cancer Study Group (IBCSG)
We are pleased to present a new “Employee Spotlight” series at the Frontier Science website. Lynette Blacher, Director of Data Management for the International Breast Cancer Study Group (IBCSG), has worked at Frontier Science for twenty years. Her educational background includes an undergraduate degree in Psychology and a Masters in Information and Library Science (MLS), both from the State University of New York at Buffalo.
FDA Approves Expanded Dosing in Neonates for Raltegravir
On November 22, the U.S. Food and Drug Administration (FDA) approved updates to the label for raltegravir (also known by the brand name Isentress). This change expands the patient population to include HIV-1 exposed full term neonates from birth to four weeks of age.
Raltegravir is a potent and selective HIV-1 integrase inhibitor. It is used as a treatment for HIV/AIDS, and can also be used as part of a post exposure prophylaxis (PEP).
The change to the label was based on data from the International Material Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) study P1110. Frontier Science, along with the Harvard T.H. Chan School of Public Health, provide data management and statistical support for this study. The quote below is from the the IMPAACT announcement.
Board member Dr. Mette Kalager Publishes Editorial on Breast Cancer Screening in BMJ
In a December 6, 2017 editorial on Breast cancer screening in The BMJ, Frontier Science board member and Associate Professor at University of Oslo Clinical Effectiveness Research Group, Dr. Mette Kalager, looks at studies analyzing data on breast cancer mortality, mammography screening, and over-diagnosis, and references a new study in the same edition at BMJ, Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ 2017;359:j5224.
New President Brings Academic and Research Experience to Frontier Science
Since its inception, Frontier Science has always viewed its statistical data management services as a collaborative effort, helping researchers and investigators understand and best utilize the data that they are using. As part of this commitment, Dr. Michael Bretthauer brings a diverse academic, research, and publication experience into Frontier Science’s core management.